Well I'm much fascinated and want to make one like this.
All I Care For - The Rich Man's Fallacy
Some people always go after wealth and wealth alone, losing all those little pleasures of human life.
Once there was a rich man who cared nothing about anything other than wealth. He bought an Audi car and was driving it along a highway to show off his new object of pride to his wealthy friends. Suddenly his car was overrun by a truck, and to his horror, the rich man noticed his car's driver side door was missing.
He was much worried about this as it would be never the same again even if he repaired his Audi. He ran to the nearby traffic police and complained bitterly about the harsh truck driver. When the policeman looked at him, he was dumbfounded and exclaimed: "But sir look down you are missing your right arm". The rich man looked down and screamed, Öh! My Rolex !"
Curious Little Cylinder Near the End of My Charger Wire. What is This?
If you own a laptop, chances are that you often wondered what was that little cylinder at the end of your charger wire.
As it is worthwhile to know what it is as it has interesting physics behind it. Perhaps or Surely you are all acquainted with what an inductor is. But for a general reader, I would like to clarify it a bit more. An inductor is a coil of wire that has the property of building up a magnetic field associated with it.
But there is a rule called Lenz law that tells you that there is no free lunch in mumbo-jumbo terms. This rule is given in the usual text book jargon below :
 |
| Cute Little Cylinder at the End of My Laptop Charger |
As it is worthwhile to know what it is as it has interesting physics behind it. Perhaps or Surely you are all acquainted with what an inductor is. But for a general reader, I would like to clarify it a bit more. An inductor is a coil of wire that has the property of building up a magnetic field associated with it.
 |
| An Inductor |
But there is a rule called Lenz law that tells you that there is no free lunch in mumbo-jumbo terms. This rule is given in the usual text book jargon below :
The direction of current induced in a conductor by a changing magnetic field due to Faraday's law of induction will be such that it will create a field that opposes the change that produced it.
So, as the inductor is reluctant to change the magnetic field associated with it, it offers a resistance to the flow of current in proportion to the rapidity with which the current changes. High frequency currents changes their polarity more frequently than their low frequency fellow currents.In essence an inductor is not well with the high frequency currents and block them then and there to a greater extent than low frequency currents. Not only coils of metal, but a ceramic material called Ferrite (which is basically rust mixed with certain other metals) surrounding a single wire can act as a sort of inductor. If AC power is supplied to a sensitive electronic device which our laptop certainly is, it is wise to surround the wire near the end of charger wire by Ferrite cylindrical shell for the following two reasons:
Credits: By Omegatron - Taken by User:Omegatron using a Canon Powershot SD110, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=3842054
So this tiny little cylindrical shell at our charger wire let to us to contemplate a bit of curious physics behind it. I am positive that this post interested you and you are eager to know more facts how physics play a central role in every piece of technology. I'm also eager like you and whenever I stumble on a good one I shall let you know that. If you have observed something curious please bring it to my attention through the comments.
- AC power lines come with spikes, i.e., sudden variation in supply voltages which passed through the SMPS (oh ! that black brick which we call charger) affect the performance of our Laptop.
- The long charger wire acts like an antenna, and due to EMI (Electro Magnetic Interference) may feed all sort of unwanted signals into our laptops motherboard circuitry.
 |
| Charger Wire or Antenna ? |
So, to avoid these interferences and improve the performance charger manufacturers add a a small cylindrical shell of Ferrite ceramic material which is called a "Ferrite Bead".This Ferrite Bead effectively isolates the laptop from unwanted high frequency current noises, but let the DC component of current charging your laptop to pass through.
 |
| Ferrite Bead with Plastic Cover Removed |
So this tiny little cylindrical shell at our charger wire let to us to contemplate a bit of curious physics behind it. I am positive that this post interested you and you are eager to know more facts how physics play a central role in every piece of technology. I'm also eager like you and whenever I stumble on a good one I shall let you know that. If you have observed something curious please bring it to my attention through the comments.
Who's This Unknown Guy?
A couple of days back I've spotted this insect crawling along the outer wall of our laboratory. Very eerie looking but I could n't identify its species then. Now after googling a bit I've found what It is. It is a House Centipede, More specifically, Scutigera coleoptrata. Literally Ä Hundred Legs". Count yourself patiently. But I suspect this lags a lot.
 |
| Mr.Hundred Legs Crawling on the Laboratory Wall |
A detailed Image from Wikipedia:
 |
| Scutigera coleoptrata |
What Sort of Idiotic Guy Who Created This Meme?
I was quite shocked to see a meme my friend recently showed which has been sent to him through WhatsApp. The dumb head who created this meme acts as if he/she does not know Newton that or actually too dumb too to have known Newton. Without Newton's Monumental Scientific work we would be living a few centuries back and as a result our dumb friend could not have either used Android smart phones or have passed it to his other dumb friend. I really pity these guys.... See this idiotic meme for yourself.
 |
| Idiotic Meme |
Nature and Nature's laws lay hid in night
God said, Let Newton be! and all was light.
 |
| Sir Isaac Newton |
 |
| Newton's Tomb At Westminster Abbey |
We should respect our Scientists, who gave us many good things (and a few bad things I agree but good things outweigh bad things by a very large margin). To disrespect such the Greatest Physicist of All Times is a sacrilege to me.
What is Happening Here? The Devil is in Details.
Really something terrible is happening. A ghost in the Subway Steps?
Maltese Cross, Rock Salt and Inter-ionic Distance - Episode I
The common salt that we use daily to make our food palatable contains a wealth of scientific information which an inquisitive mind can explore without end. The beauty and staggering amount of hierarchical complexity even of simple things is what make Physics an enchanting subject. One can't imagine how much informative even a bit of work would turn out to be in Science and especially so in Physics.
A few days ago, at the beginning of Pongal holidays, I felt like growing NaCl crystals and I believed what the heck? it should be very simple. I requested my better half for a lot of rock salt ( we call salt in impure form just taken out of sea without any further ado in Tamizh as கல் உப்பு). She looked at me askance. Then with a creepy smile I explained to her What I was going to do. Accustomed with my sudden fits of scientific inquiry she provided me with precious table salt. I took a beaker with ~150 ml potable water and boiled it bubbling hot and added the salt until the salt refused to be dissolved further (that is what is called in scientific mumbo jumbo as Saturated Solution). Well I left that beaker undisturbed for a while until all the dirt and dust settle down and poured an aliquot of that in petri dishes.
 |
| NaCl Crystal with Maltese Cross Pattern |
 |
| Another view of NaCl Single Crystal clearly Showing the Sides |
I was a bit disappointed as I expected a perfect cube but instead I got a tetragonal one. But It seems to me to be a possible habit that can be obtained by stacking unit cells of NaCl. It is nice looking with a beautiful Maltese cross pattern inside.
 |
| A Maltese Cross Batch |
How Beautiful ! But What causes this strange looking pattern. I mused over it for a while. A possible explanation is that growth of NaCl crystal is faster along edges and corners resulting in a cubic shell within shell within shell morphology and Maltese cross pattern. Well this is a sort of itching point for me. So let me step over this and go on to tell you what I did next.
I took the crystal to my department lab (Under Graduate Physics Laboratory, Dr.Zakir Husain College,Ilayangudi) and measured its dimensions using a Traveling Microscope with a least count of 0.001 cm. These dimensions are:
 |
| KAZ Measuring The NaCl Crystal Dimensions |
 |
| All the World's a Stage Including My Mobile |
Breadth x Width x Height dimensions of the crystal were measured to be 0.487 cm x 0.487 cm x 0.247 cm ( I felt a bit lazy and measured the breadth or width only and took them to be equal as the crystal seems to have a square face. But this should never be done in any serious scientific pursuit ).Then multiplying these together gave the volume of the crystal as $5.86 \times {10^{ - 8}}{m^3}$.(CAUTION: When applying numbers to any equation or formula the resulting numerical value should be rounded off to have the same number of significant digits as the least number of significant digits in the numbers we are applying. THIS IS MOST IMPORTANT and Unfortunately also MOST IGNORED. Read HERE).
Then I weighed the crystal using the digital balance in the lab.It has accuracy to only three decimal figures and when put the crystal on the weighing stage the reading danced between 0.12 g and 0.13 g.
 |
| Weighing the Crystal - Reading Danced Between 0.12 g |
 |
| and 0.13 g But Never Settled Down |
 |
(Continues...)
Forget What You Learned(or Not Learned) in High School - Mr.Carbon Can Now Have Two More Friends
This is what happens when you kick Hexamethylbenzene
strong enough:
Read more of this exotic story Here
 |
| Hexamethyl Benzene |
strong enough:
 |
| What the heck is the name of this molecule ? Could we try an IUPAC? |
Read more of this exotic story Here
The Annotated and Hyper-linked Syllabus - Atomic and Nuclear Physics - Unit I
[THIS POST IS NOW IN ITERATIVE MODE PLEASE WAIT FOR MORE SHORTLY]
COURSE CODE: 4BPH4C1
CORE COURSE VIII – ATOMIC AND NUCLEAR PHYSICS
Unit I POSITIVE RAYS
Photo electricity: Photoelectric emission – laws – Lenard’s experiment – Richardson and Compton experiment – Einstein’s photo electric equation – experimental verification of Einstein’s photo electric equation by Millikan’s experiment – Photoelectric cells.
POSITIVE RAYS
- A Historical Exposition of Positive Rays from Cambridge University with beautiful and very explanatory animations.
- A Wikipedia article on Anode Rays
- Click to download "Rays of positive electricity, and their application to chemical analyses" by J.J.Thomson, Master and Originator of the Subject. This file is in DJVU format, which has many advantages over PDF. Main advantage is it is light weight and does not consume RAM space. To view this file type, please download WinDjView program(only 2.8 MB file size) for Windows platform here. Android users just search for a suitable App in the Google Play Store of which there are plenty.
- Click to download J.J.Thomson's Paper on positive rays
 |
Anode ray tube showing the rays passing through the perforated cathode and causing the pink glow above it |
By Kkmurray - Own work, CC BY-SA 3.0,
https://commons.wikimedia.org/w/index.php?curid=14960885
What are Positive Rays?
Properties of Positive Rays
What are Positive Rays?
When a high voltage (~10000 volt) is applied between anode and perforated cathode of a gas discharge tube maintained at high vacuum (~0.001 mm of Hg), faint luminous rays are seen to extent from the back of the cathode. These rays are experimentally shown to possess positive charge. These rays are called positive rays.
1. Positive rays consist of positively charged particles.
2. The nature of these rays depends on the gas used in the discharge tube.
3. These rays travel in straight lines. They cast a shadow of the object in their path.
4. These rays get deflected by an electrical field, and bend towards the negative plate. Thus the deflection of the positive rays is in a direction opposite to that shown by the cathode rays.
5. These rays are also deflected by the magnetic fields in the direction opposite to that of the cathode rays.
6. These rays can produce mechanical as well as chemical effects.
7. The ratio of charge (e) to mass (m), i.e.,(e/m) for the particles in the positive rays is not the same for all gases.
8. The ratio e / m for the positive rays is very low as compared to the e / m value for cathode rays.
9. Positive rays affect photographic plate.
10. They can produce ionization in gases.
11. They cause fluorescence.
Specific Charge (or) Charge to Mass Ratio (e/m) of Positive Rays
Charge to Mass ratio of a charged particle serves much like a signature of that particular species of that particle. Charge and Mass along with Spin constitute key characteristics of molecular, atomic and sub-atomic particle species. If we neglect relativistic effects, which we can always do with greater confidence in discharge tube experiments, charge to mass ratio alias specific charge becomes the single most important characteristic of charged particle species determining their behavior under the action of electromagnetic field.
For Cathode e/m ratio is a constant immaterial of the gas used inside the discharge tube. This is because cathode rays consist of electrons emitted from negative electrodes, which has a fixed charge to mass ratio. On the other hand positive rays consist of gas ions and hence their charge to mass ratio depends on the gases used to fill the discharge tube. Positive rays have thousands of times less specific charge than the cathode rays.
What is a Spectrum ?
spectrum (n.) 1610s, "apparition, specter," from Latin spectrum (plural spectra) "an appearance, image, apparition, specter," from specere "to look at, view" . Meaning "visible band showing the successive colors, formed from a beam of light passed through a prism" first recorded 1670s. Figurative sense of "entire range (of something)" is from 1936.
Credit:http://www.etymonline.com
So, A spectrum is an entire range of color,charges,masses,speeds or something.
Mass Spectrum is sorting ions with respect to their masses or more particularly their Mass to Charge ratio or Charge to Mass ratio(works just as fine).
Aston's Mass Spectrograph

Francis William Aston (1 September 1877 – 20 November 1945)
- Aston won the 1922 Nobel Prize in Chemistry for his prominent work in Physics(!!?). Click Here to Download and Read Aston's Nobel Lecture.
- For Historical Details Please Visit Here.
- Click Here to read a Nice Paper on Development of Mass Spectrometers.
- Download a History Poster Here.

Aston Working With His Mass Spectrometer - Can You See the Large Electromagnet?
Bainbridges's Mass Spectrograph
1. Positive rays consist of positively charged particles.
2. The nature of these rays depends on the gas used in the discharge tube.
3. These rays travel in straight lines. They cast a shadow of the object in their path.
4. These rays get deflected by an electrical field, and bend towards the negative plate. Thus the deflection of the positive rays is in a direction opposite to that shown by the cathode rays.
5. These rays are also deflected by the magnetic fields in the direction opposite to that of the cathode rays.
6. These rays can produce mechanical as well as chemical effects.
7. The ratio of charge (e) to mass (m), i.e.,(e/m) for the particles in the positive rays is not the same for all gases.
8. The ratio e / m for the positive rays is very low as compared to the e / m value for cathode rays.
9. Positive rays affect photographic plate.
10. They can produce ionization in gases.
11. They cause fluorescence.
Specific Charge (or) Charge to Mass Ratio (e/m) of Positive Rays
For Cathode e/m ratio is a constant immaterial of the gas used inside the discharge tube. This is because cathode rays consist of electrons emitted from negative electrodes, which has a fixed charge to mass ratio. On the other hand positive rays consist of gas ions and hence their charge to mass ratio depends on the gases used to fill the discharge tube. Positive rays have thousands of times less specific charge than the cathode rays.
What is a Spectrum ?
Credit:http://www.etymonline.com
So, A spectrum is an entire range of color,charges,masses,speeds or something.
Mass Spectrum is sorting ions with respect to their masses or more particularly their Mass to Charge ratio or Charge to Mass ratio(works just as fine).
Aston's Mass Spectrograph
 |
| Francis William Aston (1 September 1877 – 20 November 1945) |
- Aston won the 1922 Nobel Prize in Chemistry for his prominent work in Physics(!!?). Click Here to Download and Read Aston's Nobel Lecture.
- For Historical Details Please Visit Here.
- Click Here to read a Nice Paper on Development of Mass Spectrometers.
- Download a History Poster Here.
 |
| Aston Working With His Mass Spectrometer - Can You See the Large Electromagnet? |
Bainbridges's Mass Spectrograph
Feynman - The Practical Man
When We aspire to become a Master in any subject it is advisable to follow the footsteps of a Master well known and Who could be a Greater Master than Richard Feynman?.
 |
| Richard Feynman |
Feynman in his quasi-autobiographical work "Surely You're Joking, Mr. Feynman! (Adventures of a Curious Character)" tells us he taught himself calculus using the book "Calculus for the Practical Man" by Thompson. Fortunately if you like a digital copy you can download it for free in the following link.
Appearance Deceives - A Story of Cats and Roosters
Once upon a time in Africa, roosters ruled
cats. The cats worked hard all day and at night they had to bring all they had
gathered for the roosters. The king of the roosters would take all the food for
himself and for the other roosters. The roosters loved to eat ants. Thus, every
cat had a purse hung round its neck, which it filled with ants for the king of
the roosters. The cats did not like the situation. They wanted to rid
themselves of the king so that the food they gathered through hard work and
great difficulty would be their own. But they were afraid of the roosters.
By Creator:Jacob Victors (www.miastokobiet.pl) [Public domain], via Wikimedia Commons
The roosters had told the cats that rooster’s combs were made out of fire and that the fire of their combs would burn anyone who disobeyed them! The cats believed them and therefore worked from early morning until night for the roosters. One night, the fire on the house of Mrs. Cat went out. She told her kitten, Fluffy, to bring some fire from Mr. Rooster’s house. When Fluffy went into the house of the rooster, she saw that Mr. Rooster was fast asleep, his stomach swollen with the ants he had eaten. The kitten was afraid to wake the rooster, so she returned home empty handed and told her mother what had happened. Mrs. Cat said, “Now that the rooster is asleep, gather some dry twigs and place them near his comb. As soon as the twigs catch fire, bring them home.”
Fluffy gathered some dry twigs and took them to the rooster’s house. He was still asleep. Fluffy fearfully put the dry twigs near the rooster’s comb but it was no use, the twigs did not catch fire. Fluffy rubbed the twigs against the rooster’s comb again but it was no use they would not catch fire. Fluffy returned home without any fire and told her mother, “The roost’s comb does not set twigs on fire.” Mrs. Cat answered “Why can’t you do anything right! Come with me I’ll show you how to make fire with the rooster’s comb.” So together they went to the house of Mr. Rooster. He was still asleep. Mrs. Cat put the twigs as near to the rooster’s comb as she could. But the twigs did not catch fire. Then, shaking with fear, she put her paw near the rooster’s comb and gently touched it. To her surprise, the comb was not hot, it was very cold, and it was just red colored. As soon as Mrs. Cat realized that the roosters had lied to the cats about their combs, she joyfully went out and told the other cats about the rooster’s tricks.
From that day on, the cats no longer worked for the roosters.At first, the king of the roosters became very angry and said to the cats; “I will burn all of your houses if you do not work for me!” But the cats said, “Your comb is not made of fire. It is just the color of fire. We touched it when you were asleep. You lied to us.”
When the king of the roosters found out that the cats knew that he had lied to them, he ran away. Now, whenever roosters see a cat, they scurry away, because to this very day they are afraid of cats.
Laws of Photoelectric Emission
1. For a given photo sensitive material, there is a minimum frequency called the threshold frequency, below which emission of photoelectrons stops completely, however great the intensity may be.
2. For a given photosensitive material, the photo electric current is directly proportional to the intensity of the incident radiation, provided the frequency is greater than the threshold frequency.
3. The photoelectric emission is an instantaneous process. i.e. there is no time lag between the incidence of radiation and the emission of photo electrons.
4. The maximum kinetic energy of the photo electrons is directly proportional to the frequency of incident radiation, but is independent of its intensity.
Complete Syllabus For Physics - Download as a PDF
To Download Complete Syllabus for Physics ( Alagappa University 2014-2017) Click the following link:
Syllabus - PDF
Why Einstein Did Not Get His Nobel Prize for Relativity ?
Read an interesting article HERE on a glitch in Einstein's Nobel Prize Citation. Einstein's Nobel Lecture had been delivered at another occasion and hence does not contain even a single reference to Photo Electric Effect. Strange !

Scanning Tunneling Microscope - Links for Netizens and Additional Material
- A Wikipedia Schematics, If you find this more appropriate and to your liking use this:
- Atom Level Resolution of Carbon atoms on a graphite surface. Let Boltzman Soul rest in Peace!
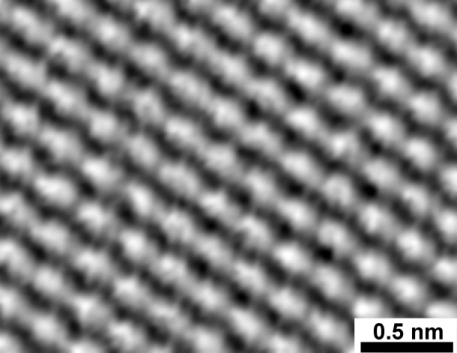
- Gold Atoms, They are not yellow of course, a suitable false color applied by the computer.
| The Quantum Coral - The Most Famous Image Produced by STM |
- See this animation video
- This is a 3 in 1 video about STM,SEM and AFM, which we shall see in future lectures.
- World's Smallest Animation Movie - Just check this animation "A Boy and His Atom" by IBM.
- And How They Moved These O-Atoms ! அணுவைத் துளைத்து ஏழ் கடலைப் புகட்டிக் குறுகத் தறித்த IBM !
- Gerd Binnig and Heinrich Rohrer were awarded Nobel Prize in 1986 for their invention of STM.
Syllabus for Fundamentals of Nanoscience
III YEAR – VI
SEMESTER
COURSE CODE:
4BPHE3C
ELECTIVE
COURSE III (C) – FUNDAMENTALS OF
NANOSCIENCE
Unit I Introduction
Introduction to
Nanotechnology – Background and definition of Nanotechnology – Nano materials –
Size Dependence.
Types: Nanowires, Nanotubes,
Quantum Dots, Nanocomposites – Properties – Ideas about Nano materials
synthesis.
Unit II Carbon
Nano Tubes (CNT)
Introduction to
CNT – SWNT – MWNT – Properties. CNT based Nano
objects- Applications.
Unit III Fabrication
Fabrication methods – Top down
processes – Milling, lithographics, Machining process. Bottom–Up process – MBE
and MOVPE, liquid phase methods, colloidal and sol – gel methods – Self
Assembly
Unit IV Characterization
Scanning Probe
Microscopy – Principle of operation – Instrumentation – Scanning Tunneling
Microscopy – STM probe construction and measurement.
Atomic Force
Microscopy – Instrumentation and Analysis – Tunneling Electron Microscopy–
operation and measurement
Unit V Nano devices
and Applications
Optical memories, Nano materials
applications in magnetism – in electronics. Sensors – in Biomedical field – in
optics – Nano layer applications – Nano particle applications
Reference
1.
Hand book of Nanotechnology – Bharat Bhushan.
2.
Nano technology and Nano electronics – W. R. Fahrner
(Editor).
3.
Materials Science – P. Mani, G. Ranganath, R. N.
Jayaprakash.
4.
Nanotechnology – Mark Ratner, Daniel Ratner.
♣♣♣♣♣♣♣♣♣♣
Subscribe to:
Posts (Atom)

















